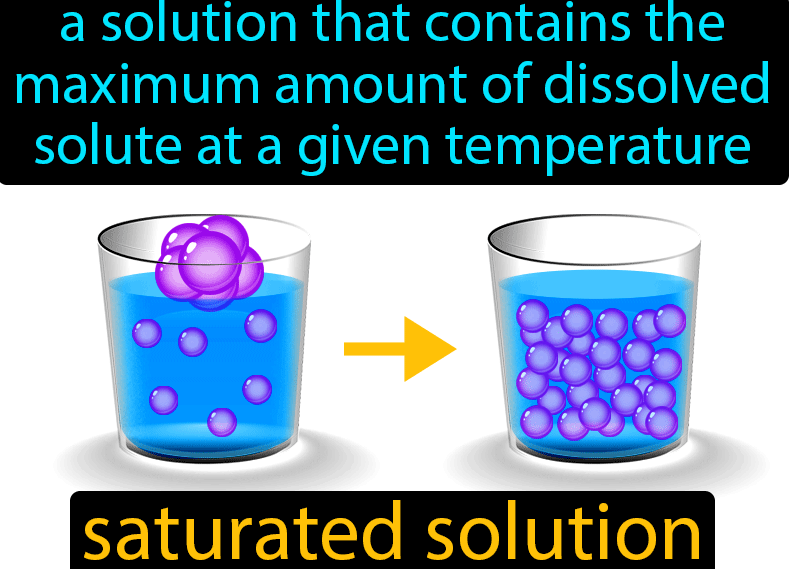
Saturated solution
A saturated solution is a solution that contains the maximum amount of solute that can be dissolved in a given solvent at a given temperature. This occurs when the chemical potential of the solute and solvent are equal. Saturated solutions are in a state of dynamic equilibrium and further addition of solute will not increase the solute concentration of the solution.
An example of a saturated solution would be a sugar solution at room temperature. If sugar is added to a glass of water, the water will become saturated when the sugar concentration reaches approximately 8.2% (w/w). At this point, any further addition of sugar will not result in a higher concentration of sugar in the solution.
Unsaturated solution

An unsaturated solution is a solution that contains less solute than can be dissolved in it. It is a solution that is not at equilibrium, and more solute can be dissolved in it. The concentration of the solution is lower than the maximum amount of solute that can be dissolved in it. An example of an unsaturated solution is sugar water, with the sugar being the solute. If you add more sugar to the water, the sugar will dissolve until the solution is saturated with sugar.
