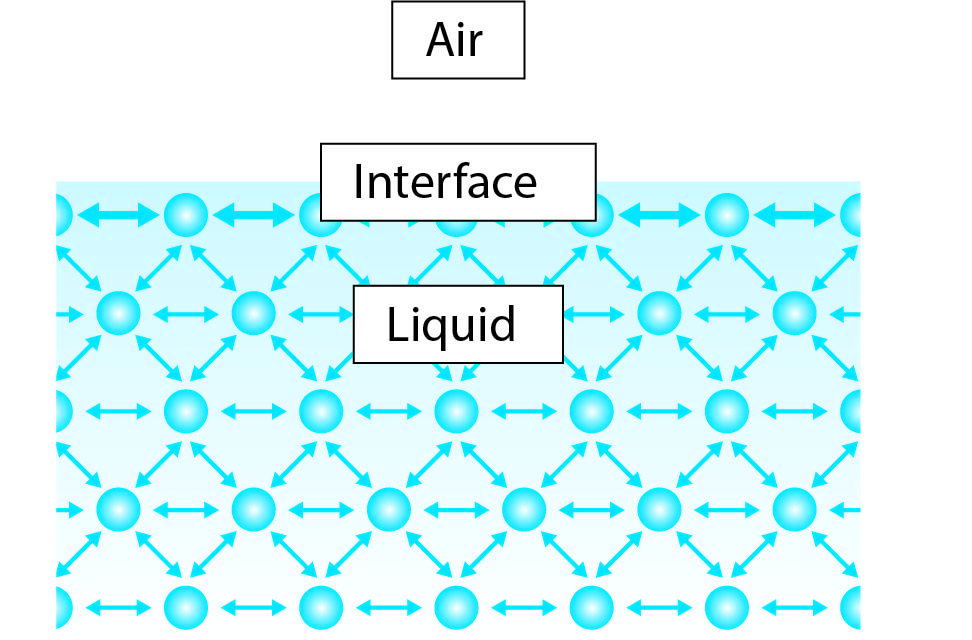
Surface tension is the phenomenon in which the surface of a liquid is strongly attracted to other similar liquids and to itself. This attraction allows the liquid to form a thin sheet or film that is highly resistant to rupture. It is caused by the cohesive forces between molecules of the same liquid. The surface tension of water is 72.8 mN/m at room temperature (20°C). The surface tension is higher for purer water and lower for more impure water. The surface tension of water helps it to form drops and bubbles and it allows insects to walk on the surface of water. It also plays an important role in the behavior of capillary action in plants.
For example, when a glass is filled to the brim with water, the water is held in the glass due to surface tension. The molecules at the surface of the water are attracted to each other and form a film-like structure on the surface. This structure is strong enough to resist the force of gravity and prevent the water from spilling out. This phenomenon is known as the meniscus.
Surface tension is the property of a liquid that enables it to form a film on its surface. The molecules on the surface of a liquid are held together by forces of attraction, which give the liquid a certain amount of tension. This tension is what allows a liquid to form droplets, which have a round, spherical shape due to the surface tension. The greater the surface tension, the higher the droplet will be and the more spherical its shape will be.