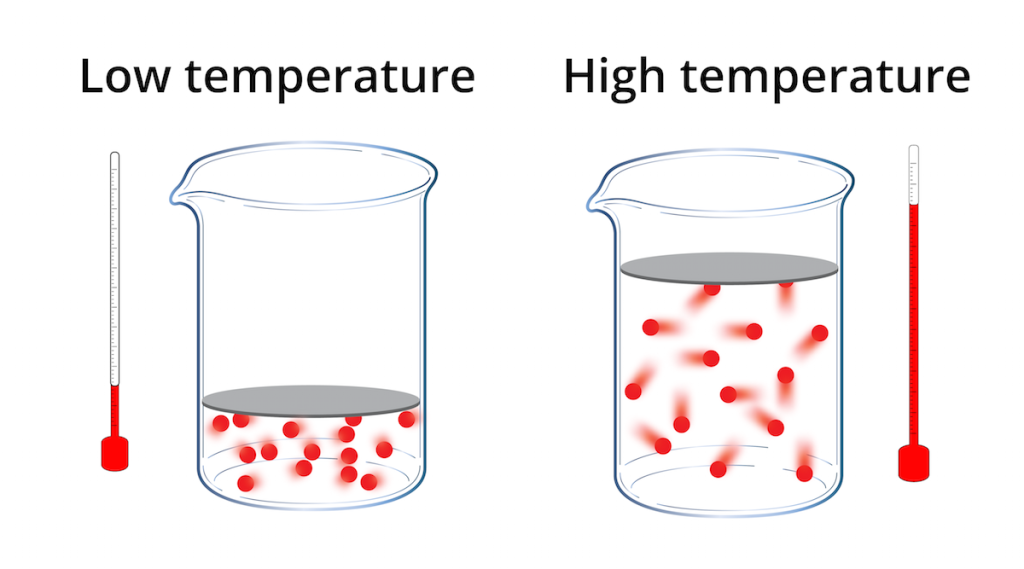
The temperature of a gas is determined by measuring the average kinetic energy of its molecules. This is done by measuring the pressure, volume and amount of the gas present. The temperature of a gas is closely related to the average kinetic energy of its molecules, and increases as the average kinetic energy increases.
