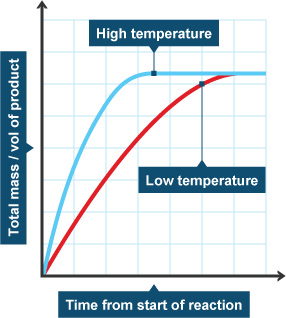
The temperature and rate of reaction is directly related. An increase in temperature will typically result in an increased rate of reaction. This is due to the fact that molecules move faster and with more energy when exposed to higher temperatures, which increases the chances of successful collisions between reactants. For example, an increase in the temperature of an acid-base reaction will result in increased rate of the reaction.
